Learn about who we are, what we believe
and the people who lead us
Discover our unique scientific approach
for diseases with high unmet need
The first and only FDA-approved
treatment for Demodex blepharitis
Explore the possibilities of our robust pipeline
to address significant unmet patient needs
Find additional corporate
and stock information
TP-04: An investigational therapeutic for ocular rosacea1
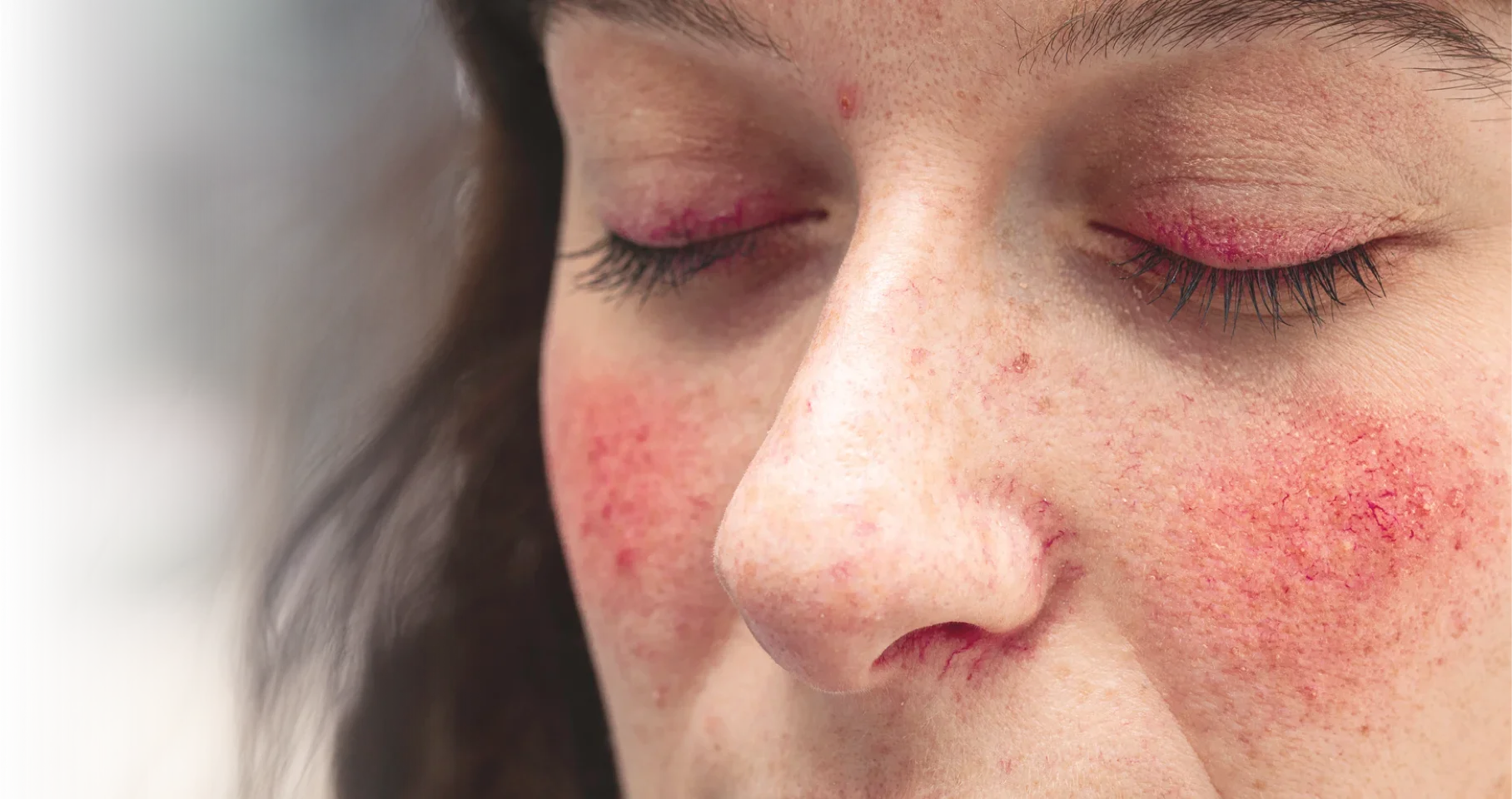
Actual representation of ocular rosacea.
TP-04 is a sterile, topical ophthalmic, gel formulation of lotilaner in development for periorbital and eyelid application for potential treatment of ocular rosacea. Lotilaner is a well-characterized antiparasitic agent that paralyzes and kills Demodex mites by selectively inhibiting parasite-specific GABA-chloride channels.1
Approximately 15-18 million people in the United States are affected by ocular rosacea,2,3 a highly prevalent inflammatory condition of the eye.4
GABA=gamma-aminobutyric acid.
1. Data on file. Tarsus Pharmaceuticals, Inc. 2. Dudee J, Ing EB (ed). Ocular rosacea. Updated March 13, 2024. Accessed January 6, 2025. https://emedicine.medscape.com/article/1197341-overview#showall 3. U.S. and world population clock. United States Census Bureau. Accessed January 6, 2025. https://www.census.gov/popclock/ 4. Macsai MS, Mannis MJ, Huntly AC. Chapter 41: Ocular rosacea. Published 1996. Accessed January 6, 2025. https://escholarship.org/content/qt2x84c8cp/rosacea.html
Our Terms of Use and Privacy Policy have changed.
By clicking accept, you agree that you have read and will abide by these new terms.